A Primer on Atom Trap Trace Analysis (ATTA)
For colleagues who wish to apply radio-krypton or radio-argon dating
Atom Trap Trace Analysis (ATTA) is a laser-based atom-counting method capable of analyzing environmental isotope tracers 85Kr, 39Ar, and 81Kr, each covering a distinct age range around the respective half-life (Table 1). Combined with 14C, the tracers can be used to probe events in the age range from a few years all the way to 1.3 million years (Fig. 1). The noble-gas tracers have ideal geophysical and geochemical properties that simplify data interpretation; they have well determined, near uniform distributions in the atmosphere, and relatively simple transport processes underground. These isotopes are now being used to trace ocean circulation, date glacier ice, and trace groundwater pathways and help determine the recharge rates of aquifers around the world.
List of ATTA-related publications(Please help us update the list by contacting tangr2018@ustc.edu.cn)
Table 1. Long-lived noble-gas isotopes in the environment
| Isotope | Half-life (year) | Effective age range (year) | Atmospheric isotopic abundance | Primary Production mechanism |
|---|---|---|---|---|
| 85Kr | 10.76 ± 0.02 | 2 – 55 | 2 × 10-11 | Nuclear fuel reprocessing |
| 39Ar | 269 ± 3 | 55 – 1,400 | 8 × 10 -16 | Cosmogenic |
| 81Kr | 230,000 ± 11,000 | 40,000 – 1,300,000 | 6 × 10-13 | Cosmogenic |
Fig. 1 The effective age ranges covered by radioactive gas isotopes. 14C stays in the atmosphere in the form of CO2 gas.
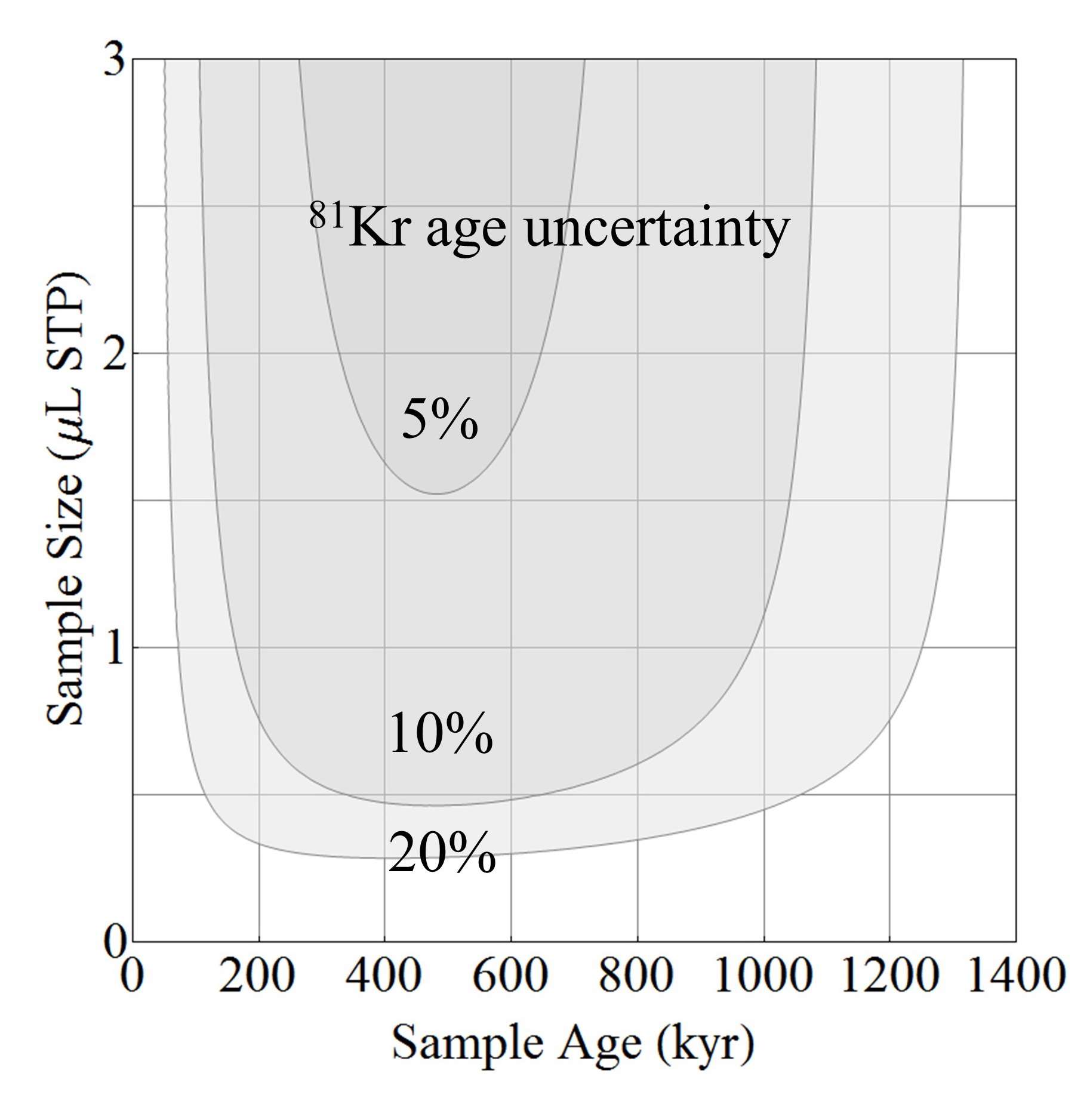
Fig. 2 Sample size vs. sample age in 81Kr dating for a targeted age precision of 5%, 10%, or 20%.
The operation of analyzing 85Kr, 39Ar, 81Kr in an environmental sample consists of three steps:
1) Sampling – A degassing instrument is used to extract gas dissolved in water or trapped ice. Groundwater degassing is usually done in the field. Ice (or water) can also be brought to a lab for degassing.
2) Purification – krypton and argon gases are separated from the bulk gas samples using cryogenic adsorption and chemical reaction techniques.
3) Counting – The purified krypton (or argon) sample is injected into an atom-trap apparatus for 81Kr- and 85Kr-counting (or 39Ar-counting).
Age is calculated based on the measured isotopic abundances. For example:
Step 1. Sampling
The amount of krypton (or argon) sample needed to achieve a certain age precision depends on both the sample size and the age itself (Fig. 2). Typically, bulk gas contains ppm level of krypton and percent-level of argon. A sample of 1 micro-liter (STP) of krypton can be extracted from approximately 20 kg of water or 8 kg of Antarctic ice (more for mountain glacier ice). A sample of 1 milli-liter (STP) of argon can be extracted from approximately 2 kg of water or 3 kg of mountain glacier ice. The recommended sample sizes are listed in Table 2.
It is critical that sampling is performed without any mixture of leaked air containing modern krypton (or argon). For this reason, specialized equipment and procedures are developed.
Groundwater is usually degassed in the field as it is more convenient to ship a small gas cylinder or a gas bag (Fig. 3a) instead of water. Fig. 3b shows a degassing instrument. As water flows through a membrane in the instrument, the dissolved gas is separated and compressed into a gas cylinder or a bag. Typically it takes less than an hour to sample a well. Since groundwater sample is relatively easy to obtain, we recommend a somewhat larger sample size.
Glacier ice samples are typically shipped back to a storage lab, where an ice melter is used to extract the gas content (Fig. 4). It is possible to bring an ice melter to the field for on-site gas extraction.
Seawater is sampled via Niskin bottles hung on a CTD. Once raised onto the deck, the water needs to be transferred to a gas-sealed tank for storage (Fig. 5). Later, the tanks containing seawater samples are sent to a lab for gas extraction, a process similar to the groundwater case. Alternatively, gas extraction can be carried out on the ship so as to save the number of tanks, storage space, and shipping cost.Table 2. Sample size requirement
Isotope Groundwater Sea Water Mountain Glacial Ice Polar Ice 85Kr 20 kg 4 kg 3 kg 1 kg 39Ar 10 kg 4 kg 2 kg 1 kg 81Kr 20 kg -- 3 kg 1 kg List of groups capable of field sampling of groundwaterFig. 3. (a) Gas bag (10 L size) and gas cylinder (4 L size) for storing and shipping bulk gas samples. (b) Groundwater degassing instrument.
Fig. 4. Ice melter, for extracting gas content of an ice sample.
Fig. 5. Transfer seawater sample from a Niskin bottle to a storage tank, performed by Fei Teng of the First Institute of Oceanography of China.
Step 2. Purification
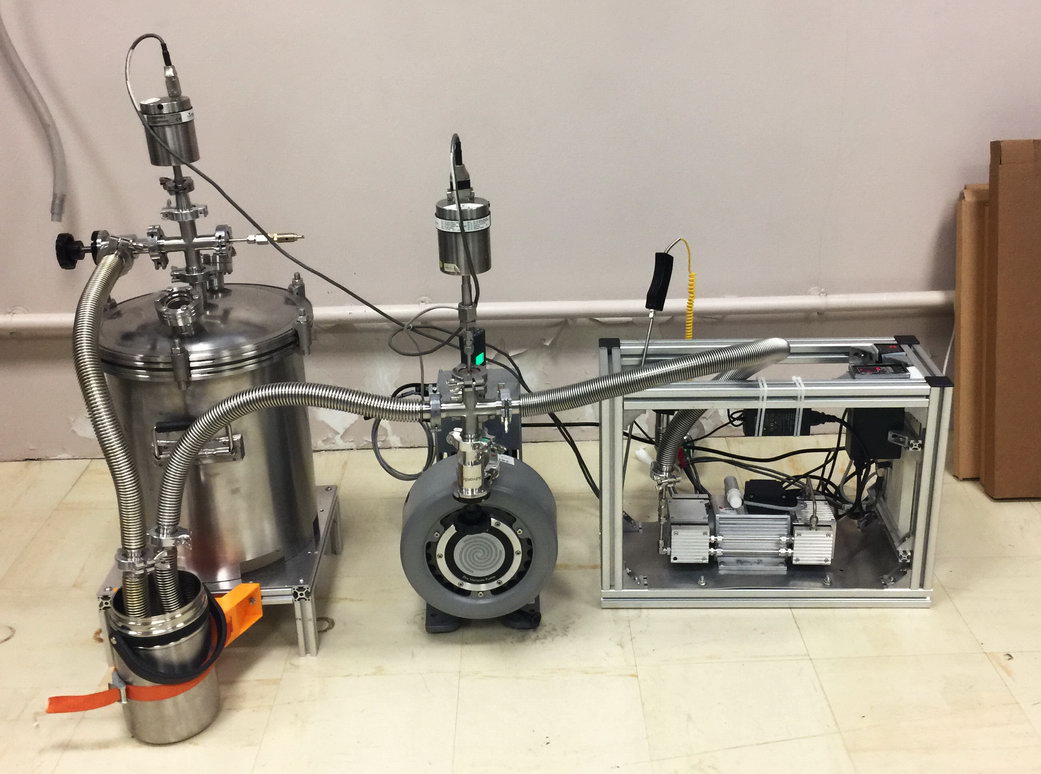
A purification step is needed to extract the ppm-level krypton (or the percent-level argon) from the bulk gas sample. Krypton and argon can be separated from the same bulk gas sample so that, if needed, one bulk sample can be used for the analysis of all these three isotopes This is done with an apparatus employing cryogenic adsorption, chemical reaction, and gas chromatography techniques (Fig. 6). In the purified sample, krypton (or argon) concentration is preferred to be > 50%.List of groups capable of sample purificationFig. 6. Schematic of the purification process used to extract krypton from bulk gas (Yokochi R., 2016).
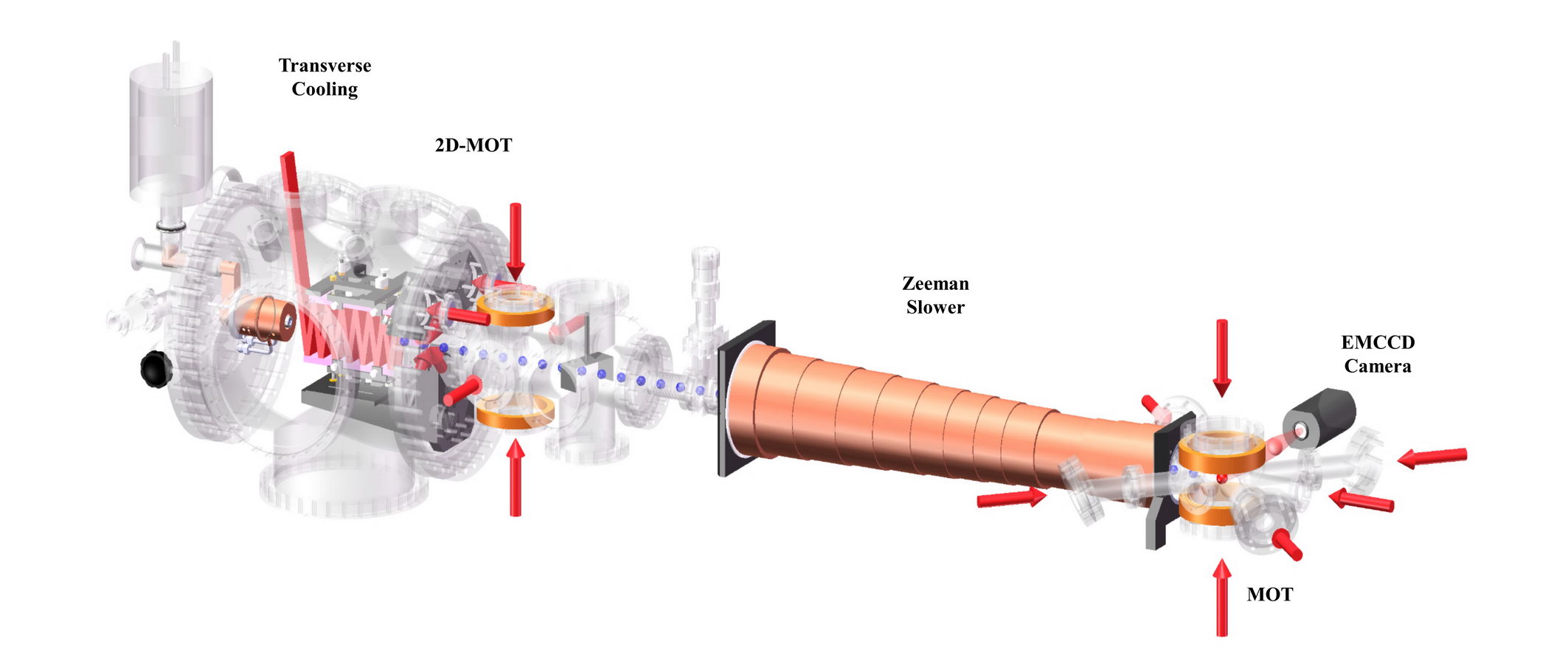
Fig. 7. An ATTA apparatus. The total length of the atomic beam-trap is about two meters. Lasers and optics are located on an adjacent laser table of a similar length.
Step 3. Atom Counting
Following purification, the krypton (or argon) sample is injected into the trap apparatus for atom counting (Fig. 7). In the Atom Trap Trace Analysis (ATTA) method, a neutral atom of a particular isotope is selectively captured by a magneto-optical trap and detected by observing its fluorescence. When the laser frequency is tuned to the resonance of the desired species, 85Kr, 39Ar, 81Kr, only atoms of this particular isotope are captured into the trap. The high degree of redundancy built into the trapping and detection process, in the form of repeated resonant excitations, guarantees that the identification of the targeted isotope is never in error. ATTA is unique among all trace analysis methods in that its detection is free of interference from any other isotopes, elements, or molecules.
List of groups with ATTA instruments