Dating based on 85Kr, 39Ar and 81Kr
For colleagues who wish to apply radio-krypton or radio-argon dating, please see Primer on Atom Trap Trace Analysis.
Welcome to visit our laboratory
Radiokrypton and radioargon dating
Radioactive isotope tracers are natural clocks in the environment. Once gas exchange ceases between a sample and the atmosphere, the abundance of the tracers contained in the sample decreases over time due to radioactive decay. As a result, the remaining abundance can be analyzed to derive the geological age of the sample, based on which the transport and evolution of the sample can be studied. This basic technique has wide applications in the earth and environmental sciences.
Long-lived noble-gas isotopes form an ideal group of tracers for the study of environmental water samples, including groundwater, ocean water, and polar ice. Approximately 98% of the noble-gas isotopes resides in the atmosphere (for 14C, it’s only 2%) for a long time, comparable to their radioactive lifetimes, achieving uniform distribution over the earth’s atmosphere. Consequently, the interpretation of the ages determined by these noble-gas isotopes is relatively straightforward. There are three long-lived noble-gas isotopes in the environment: 85Kr, 39Ar, and 81Kr. They possess lifetimes of different orders of magnitude. Together with 14C, they cover an age range from a few years to 1.3 million years (Fig. 1). Routine analyses of these isotopes would greatly boost applications in hydrology, oceanography, glaciology, and other branches of earth and environmental sciences.

Fig.1
While the analyses of stable noble-gas isotopes can be accomplished using mass spectrometry, the analyses of 85Kr, 39Ar, and 81Kr has been quite challenging, mainly due to their extremely low isotopic abundances in the range of 10-16 ~ 10-11. For example, one liter of modern surface water contains only 1,000 81Kr atoms, 8,000 39Ar atoms, and 40,000 85Kr atoms.
Atom Trap Trace Analysis (ATTA)
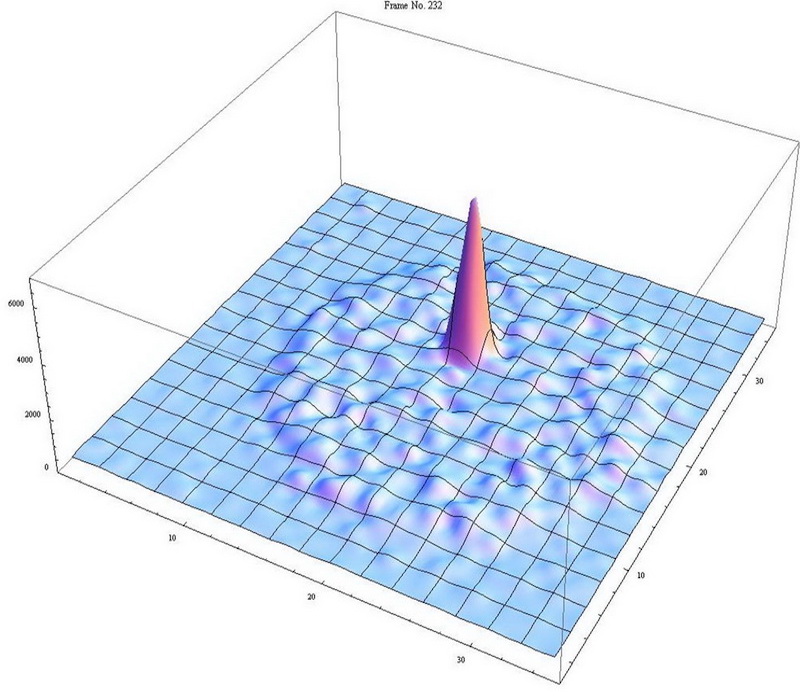
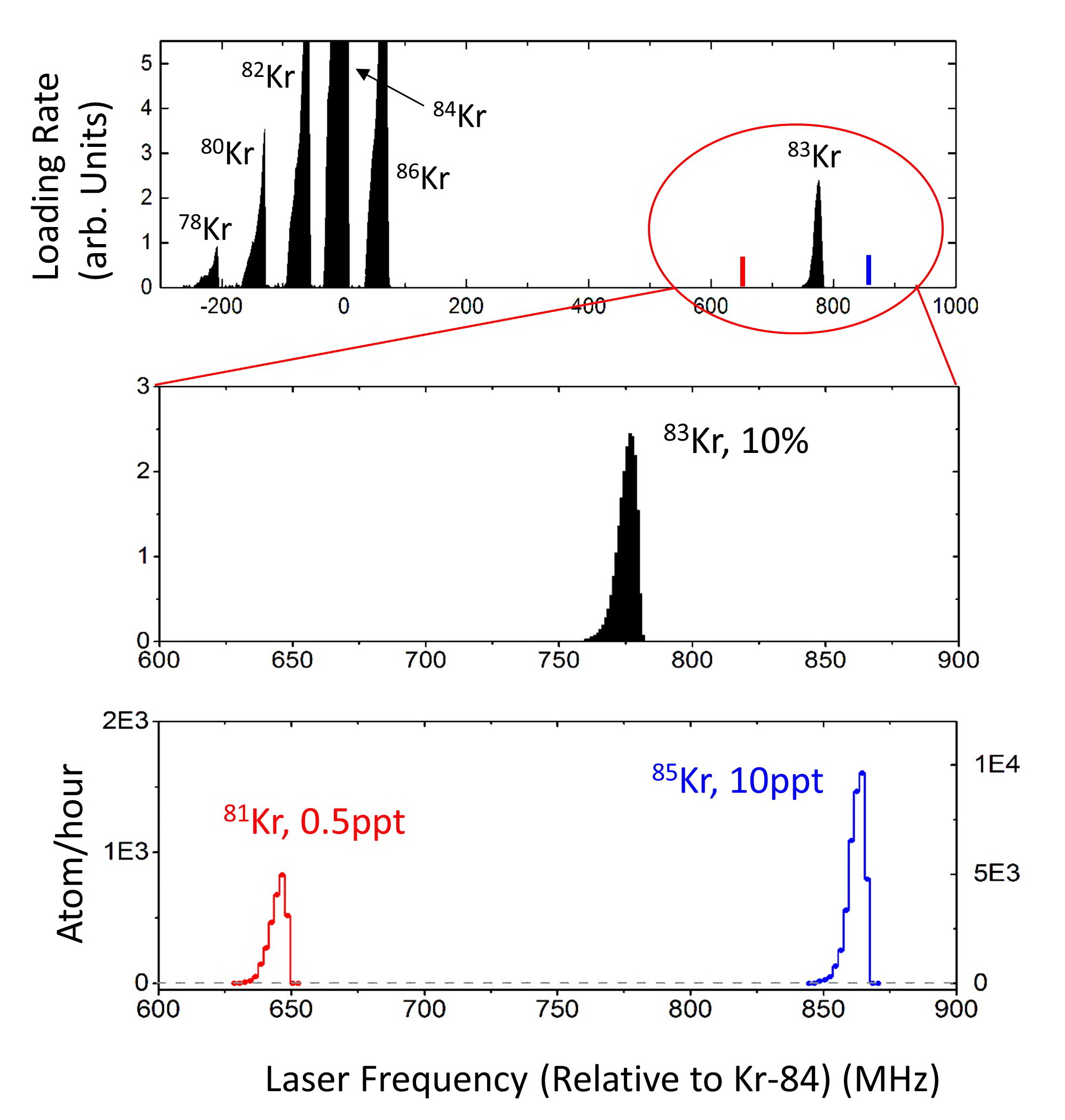
We developed the Atom Trap Trace Analysis (ATTA) method, and used ATTA to realize the analyses of 85Kr, 39Ar, and 81Kr isotopes in environmental samples (Ref. 1-4). ATTA is based laser manipulation of neutral atoms to selectively capture and detect individual atoms of the desired isotope. It achieves the required sensitivity, selectivity, and efficiency by employing a variety of techniques in atom optics, laser trapping and cooling, among which the magneto-optical trap (MOT) plays a central role. For example, when the laser frequency is tuned to the resonance of 81Kr, only 81Kr atoms can be trapped in the MOT. By measuring the fluorescence of the trapped atom, the trapped 81Kr atom can be detected with certainty (Fig. 2). On the other hand, if the laser frequency is tuned away from resonance by as little as ~ 10 MHz, the 81Kr trap ceases to work, and the counting rate drops to zero. Even though the nearby stable isotope 83Kr is more abundant by a factor of 1012, it causes no interference at all to the 81Kr signal (Fig. 3). This is a unique, advantageous property of ATTA.
By counting atoms of the desired isotope over a fixed period, and comparing the counts with that of a stable isotope, the isotopic abundance can be derived. For better detection efficiency, laser-manipulations techniques are used to achieve a high-efficiency, fast loading of the MOT. The MOT simultaneously possesses the ability for fast loading (1012/s for 84Kr) and single-atom detection.
At present, ATTA is the only technique thought to be capable of routine analysis of 85Kr, 39Ar, and 81Kr in environmental samples.
Applications
85Kr,39Ar, and 81Kr tracers have a wide range of applications in the earth and environmental sciences. Examples are:
- Groundwater resource management
- Dating of polar ice and mountain glacier ice
- Mapping of ocean currents
- Groundwater safety evaluation of nuclear waste repositories
For details of applications, please see the review article in Earth-Science reviews 138, 196 (2014)
Here are examples of collaborative projects using ATTA analyses: